this post was submitted on 25 Jul 2024
351 points (96.8% liked)
Science Memes
11404 readers
1324 users here now
Welcome to c/science_memes @ Mander.xyz!
A place for majestic STEMLORD peacocking, as well as memes about the realities of working in a lab.

Rules
- Don't throw mud. Behave like an intellectual and remember the human.
- Keep it rooted (on topic).
- No spam.
- Infographics welcome, get schooled.
This is a science community. We use the Dawkins definition of meme.
Research Committee
Other Mander Communities
Science and Research
Biology and Life Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- !reptiles and [email protected]
Physical Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
Humanities and Social Sciences
Practical and Applied Sciences
- !exercise-and [email protected]
- [email protected]
- !self [email protected]
- [email protected]
- [email protected]
- [email protected]
Memes
Miscellaneous
founded 2 years ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
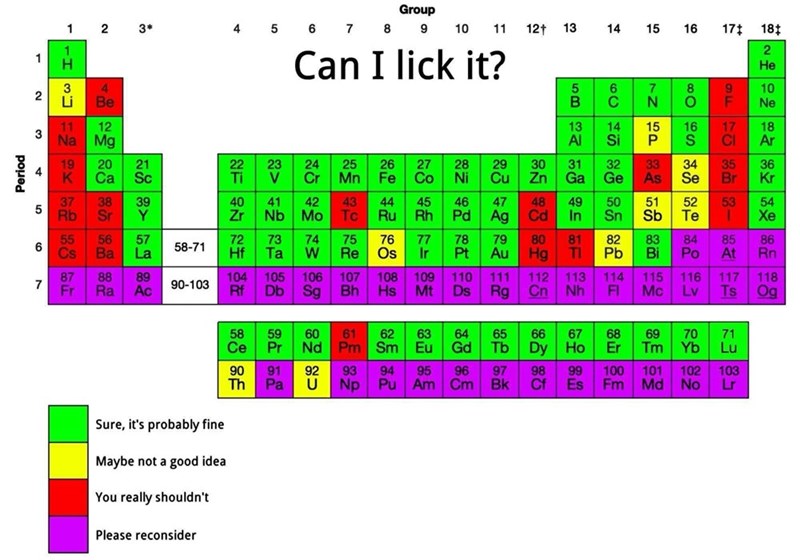
i'm not a chemist but is this licking the most common molecule form or the atomic variety
O₂ is safe but i don't think O is
I think it's framed in the context of: "How dangerous would a single molecule be to a human?". In that context, I would say
Ois safe, only because our body naturally destroys the radical oxygen molecules every day that we create with our anti-oxidants.True, in a larger quantity than our body can handle, it's extremely toxic; but a single molecule would probably not be too bad.
But I do agree, it shouldn't be Green. It should be Yellow at least.
O would completely destroy you in lickable quantities. I think you underestimate how extremely reactive it is. Just remember that it is so reactive that it reacts with oxygen to form ozone. This is not a little byproduct in extremely small quantities all throughout the body, which is also not the O radical anyway.