this post was submitted on 25 Jul 2024
351 points (96.8% liked)
Science Memes
11399 readers
1284 users here now
Welcome to c/science_memes @ Mander.xyz!
A place for majestic STEMLORD peacocking, as well as memes about the realities of working in a lab.

Rules
- Don't throw mud. Behave like an intellectual and remember the human.
- Keep it rooted (on topic).
- No spam.
- Infographics welcome, get schooled.
This is a science community. We use the Dawkins definition of meme.
Research Committee
Other Mander Communities
Science and Research
Biology and Life Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- !reptiles and [email protected]
Physical Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
Humanities and Social Sciences
Practical and Applied Sciences
- !exercise-and [email protected]
- [email protected]
- !self [email protected]
- [email protected]
- [email protected]
- [email protected]
Memes
Miscellaneous
founded 2 years ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
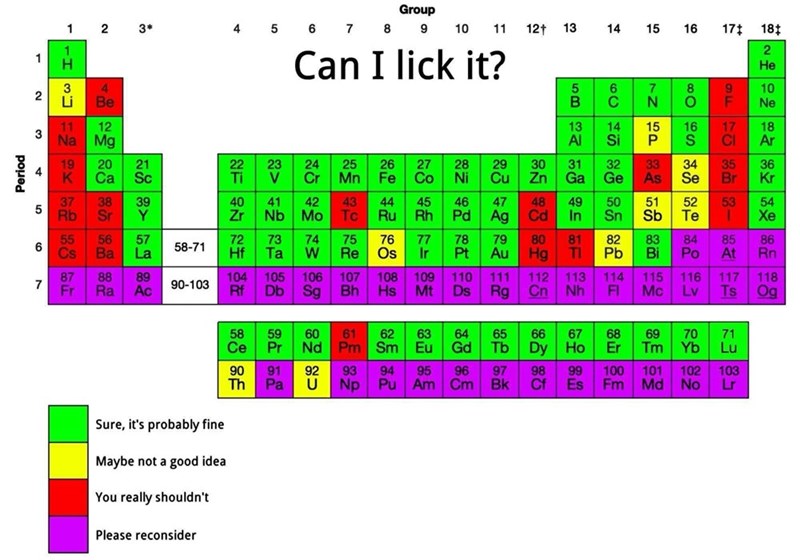
I'm pretty sure that licking pure magnesium would make your tongue explode too.
I would not be willing to lick calcium, too
Definitely not licking pure lithium, sodium, or any of the alkali (s-block) metals. My tongue is wet. That shit explodes in water, yo.
I wonder if you'd get a sort of leidenfrost effect limiting the extent of damage.
I'm not going to test that though.
Magnesium is fine (see response above). https://invidious.darkness.services/watch?v=Q_4I30Nz_b0 Just don't vomit on it before you lick it, 'cause it'll get spicy with acid.
Mg is an alkaline earth metal, not an alkali metal. :). Still have zero desire whatsoever to eat elemental Mg.
But I did say s-block didn’t I. That’s on me, I set the bar too low.
Yeah, the only reason I replied was because you were responding to the calcium dude above, then said "s-block". Just wanted to spread the good word of the 9th-most abundant element in the universe 🙏
Frankly I’m amazed I even got as much of that right as I did. It’s been more than 20 years since I took a chemistry class—a lot of them—but still. It’s been a minute.
I have elemental magnesium (4 ~50g ingots, I keep it in my library in a barely-sealed ziplock). it's shelf stable and doesn't react violently with water. Want me to try licking it and let you know? (hint: at worst it'll make a minuscule amount of milk of magnesia)
ETA: Would I stick my tongue in pyrophoric magnesium powder? No, and you wouldn't do that with pyrophoric aluminum or zinc powders, either, but that doesn't stop me from using (or licking) alumnum foil. Proof: https://invidious.darkness.services/watch?v=Q_4I30Nz_b0