this post was submitted on 22 Apr 2024
810 points (99.3% liked)
Science Memes
12250 readers
4078 users here now
Welcome to c/science_memes @ Mander.xyz!
A place for majestic STEMLORD peacocking, as well as memes about the realities of working in a lab.

Rules
- Don't throw mud. Behave like an intellectual and remember the human.
- Keep it rooted (on topic).
- No spam.
- Infographics welcome, get schooled.
This is a science community. We use the Dawkins definition of meme.
Research Committee
Other Mander Communities
Science and Research
Biology and Life Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- !reptiles and [email protected]
Physical Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
Humanities and Social Sciences
Practical and Applied Sciences
- !exercise-and [email protected]
- [email protected]
- !self [email protected]
- [email protected]
- [email protected]
- [email protected]
Memes
Miscellaneous
founded 2 years ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
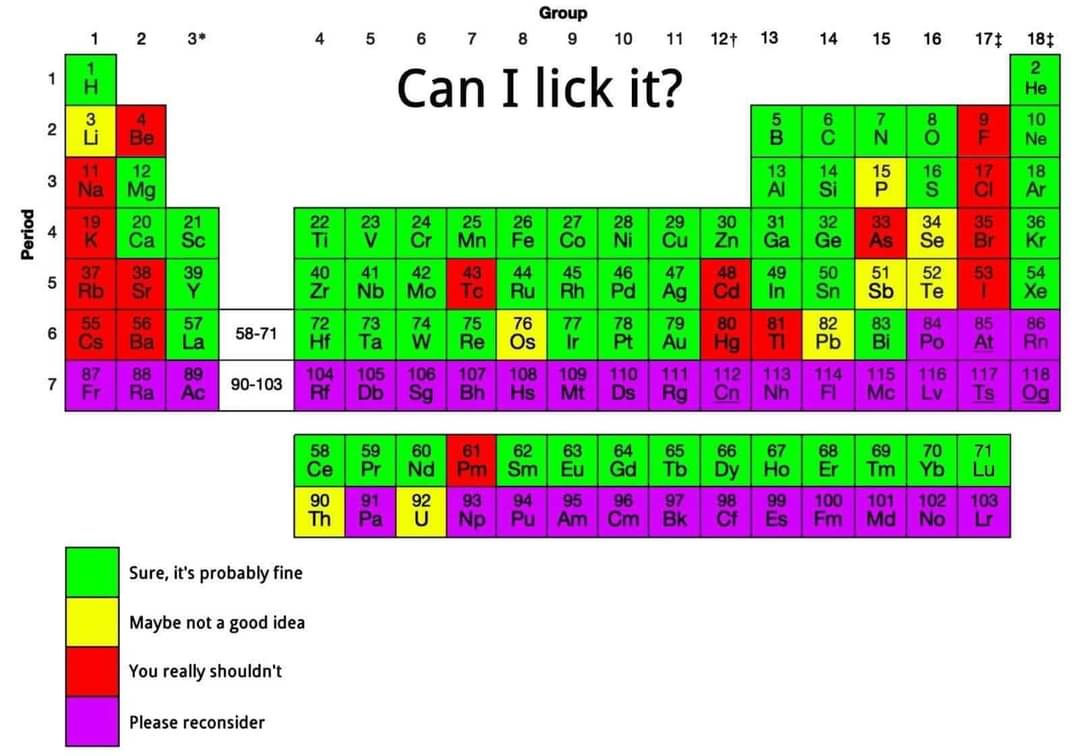
Please don't lick elemental hydrogen.
Out of curiosity, what would happen if you do?
In the hypothetical, if one were able to lick elemental hydrogen in its atomic, rather than molecular form, it would have a few potential effects. The one that would concern me most would be its aggressive reactivity, ripping hydrogens away from anything that it could in order to achieve stability. This would potentially cause tissue damage both from the deprotonation and shift in pH.
What would cause the shift in pH? The atomic hydrogen would rip off H· radicals, not H^+^ ions.
It would be more likely a secondary or tertiary effect. That is, H• radicals ripped away from their parent molecules would leave •OH, •R, and •RNH radicals. These are unstable and highly reactive, "desiring" to have that stable electron configuration. Likely, this will result in electrons being shifted to bring in more stable species, like OH-. Overall, we're looking at effectively a deprotonation of the saliva, with extra intermediary steps to stabilize the radicals.
Interesting. Given that H• is a neutral species, what would cause the preference for the creation of stable negative species (freeing up H+) over the creation of stable positive species (freeing up OH-)?
Neutral as far as pH is concerned, yes. However, radicals tend to be very reactive due to their valance not being full. I am a bit rusty, TBH, as I'm about a decade and a half out of uni but, the best way to predict the products of the reaction is to look at the high-level of the equation:
H• (excess) + H••OH + H••R + H••N-R -> H2(g) + •OH + •R + •N-RAll of the products of the initial reaction here are radicals except for the H2 molecules. They all are going to further react to form more stable species with full valances, with possible exception being the molecular hydrogen. Because the elemental hydrogen is introduced as a radical rather than protons (H+ ions) in the solution, the final products are likely to be more negatively charged, neutral, and/or have some interesting hydrogen additions, especially in the hydrocarbons and amino acids.
For example, there could be reactions like:
R• + •OH + •N-R -> R-OH + HO-N-ROverall, however, the amount of free hydrogen/protons is likely to be reduced as they are effectively removed from solution as hydrogen gas.
This is the part I don't understand. If charge is conserved, why would there be a preference for a particular charge in the products?
No. I think that you're absolutely correct. The products should have charge conserved. After initial attack of hydrocarbons by H• radicals, H2 is likely to be a significant product. Supposing STP, it would likely remove itself from solution, leaving the fresh radicals to chain react and probably making interesting and unhealthy things.
My apologies, I'm out of the lab and field 15 years now so, do make some pretty basic mistakes at times.
That makes sense.
No worries! I've enjoyed this discussion!
Nothing, because you can have only one atom of it. Multiple will just form molecular hydrogen H2. That one hydrogen atom will aggressively rip of another hydrogen of a molecule of water for example, but it won't be noticeable.