this post was submitted on 11 Jul 2024
961 points (98.8% liked)
Science Memes
11081 readers
3250 users here now
Welcome to c/science_memes @ Mander.xyz!
A place for majestic STEMLORD peacocking, as well as memes about the realities of working in a lab.

Rules
- Don't throw mud. Behave like an intellectual and remember the human.
- Keep it rooted (on topic).
- No spam.
- Infographics welcome, get schooled.
This is a science community. We use the Dawkins definition of meme.
Research Committee
Other Mander Communities
Science and Research
Biology and Life Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- !reptiles and [email protected]
Physical Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
Humanities and Social Sciences
Practical and Applied Sciences
- !exercise-and [email protected]
- [email protected]
- !self [email protected]
- [email protected]
- [email protected]
- [email protected]
Memes
Miscellaneous
founded 2 years ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
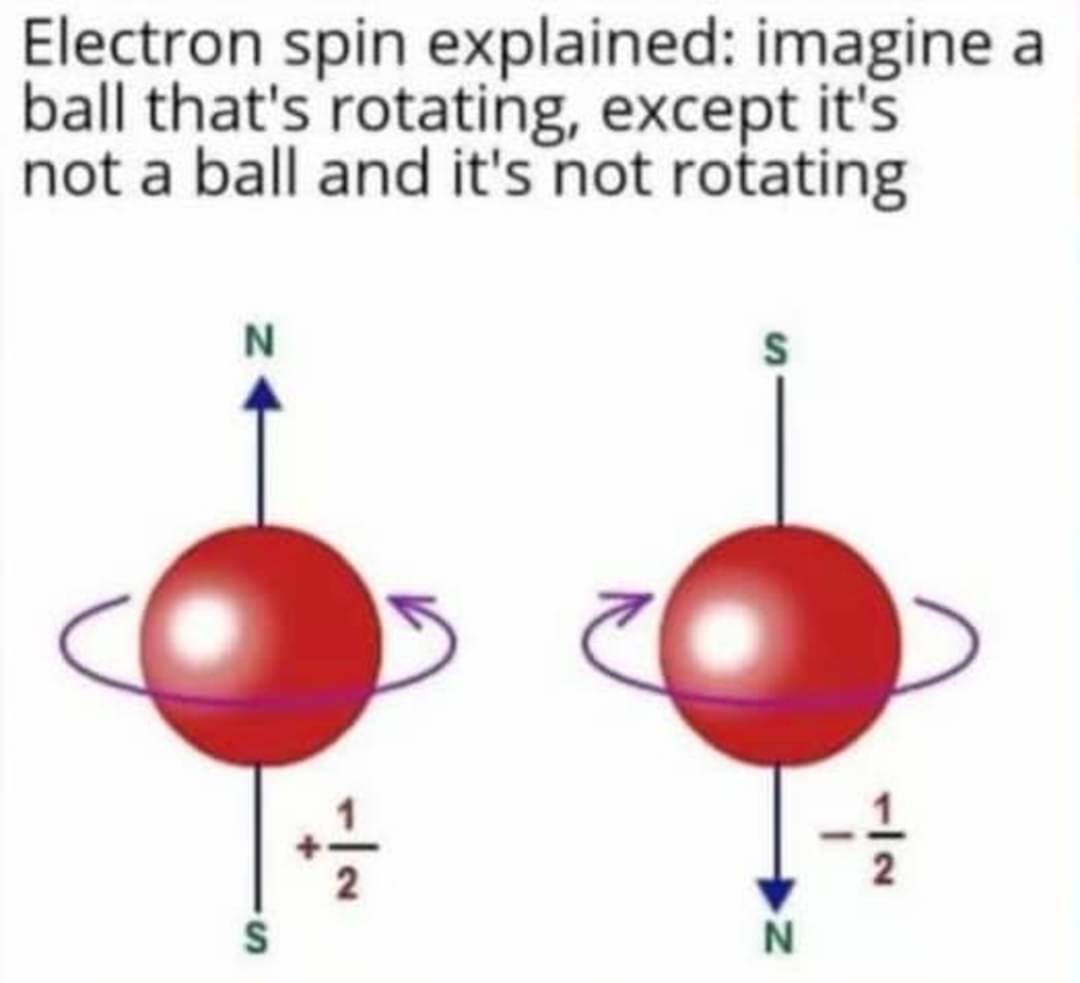
Google "Electron Orbitals". All the spaces there are all the ~~possible~~ highest likely locations for the electrons. Good Introduction to some Quantum Mechanics 👍
No! I will not relive the horrors of that chemistry class again... you can't make me. I am happily an aerospace engineer now where I don't need this chemistry nonsense, or quantum mechanics.
Ah let's see, of the top of my head...
~~1s² 2s² 2d⁶ 3s² 2p¹⁰ ...~~
Edited (iirc now, the d block is in the middle with the transition metals, p block with metallics, Halogens, Noble Gases...):
1s² 2s² 2p⁶ 3s² 2d¹⁰ ...
This I was fine with. But that fake make believe redox math? Like are all chemist bad at actual math, so they just came up with their own fake version?