TCE
Budanov, Head of Ukrainian Intelligence, now reverses his stance and claims that the threat of “artificial catastrophe” at the ZNPP is “quietly decreasing”.
In other words, he called off the attack.
What changed?
CARBIDE DISSOLUTION RATE AND CARBIDE CONTENTS IN USUAL HIGH ALLOYED TOOL STEELS AT AUSTENITIZING TEMPERATURES BETWEEN 900 ◦C AND 1250 ◦C
S. Wilmes and G. Kientopf Uddeholm GmbH Düsseldorf Deutchland
Abstract The wear resistance of cold work steels essentially depends on the amount and on the types of undissolved carbides present in the hardened condition. The aim of this paper is to show the differences of carbide content in tool steels for cold work used today. Only the content of undissolved carbides after heat treatment is important for wear resistance. Therefore the changes of carbide content by heat treatment have been determined. The rate of carbide solution at austenitizing temperature and the time to reach equilibrium structure were also tested.
Source via Bohler: https://www5.kau.se/sites/default/files/Dokument/subpage/2010/02/39_533_547_pdf_17397.pdf
Mirror: https://t.me/carbide_microstructure/235
For reference, in this document, X190 CrVMo 20-4 is m390.
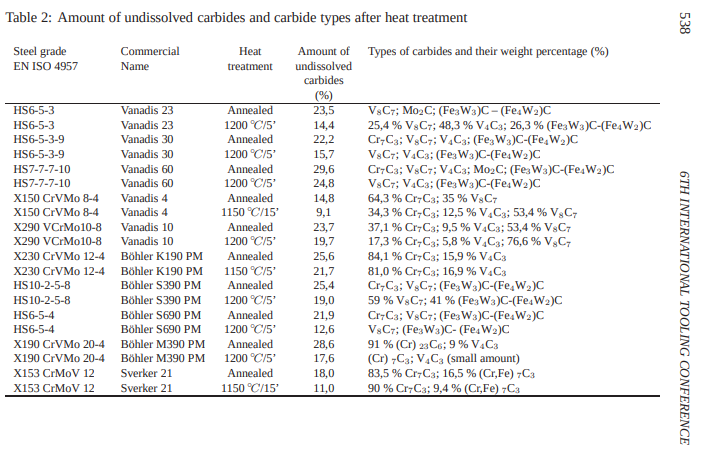
Table 2 shows us in an annealed state, the amount of undissolved carbide percent is 28,6% with 91 % (Cr) ~23~ C~6~ and 9 % V~4~C~3~
When heat treated 1200◦C @ 5 mins the amount of undissolved carbide percent is 17,6% (Cr) ~7~C~3~ and V~4~C~3~ (small amount).
When the amount of carbides drops, normally the wear resistance should also decrease. But one also has to take into account the type of carbide, that means the hardness of carbides. Table 2 gives some information of the carbide types to be found in the annealed and in the at 1150◦C or 1200◦C hardened condition. Table 2 also shows how the types of carbides and the weight percentage of the carbide types are changed by the heat treatment. The weight percentage of the stable carbide type MC increases whilst the content of the less stable carbide types M~23~C~6~ or M~7~C~3~ is reduced, changed to more stable carbide types or are completely dissolved.
This shows the amount and the types of carbides, which explains why some steels have a higher wear resistance than others. The paper gives examples, and you should give it a good read.

source: https://knifesteelnerds.com/2019/07/15/carbide-types-in-knife-steels/
We can gather 2 things with this information. There is approx 39.5% less undissolved carbides which provides more available C and Cr in solution for hardness and stain resistance (This can be very useful when using secondary hardening). But also the weaker Cr~23~C~6~ carbides are gone, allowing more C and Cr in the solution. Now we have K2 Cr carbides, the hardest Cr carbides and MC V carbides which are even harder than Cr carbides. K2 carbide is about 79 on the Rockwell C scale, compared to 72 for the K1 carbide.
Cr~23~C~6~ carbides are not as hard as the other's and they bind a lot of C and Cr in solution, and they are responsible for intergranular corrosion.
Via: Metallurgy of Steel for Bladesmiths & Others who Heat Treat and Forge Steel By John D. Verhoeven (2005) Source: https://archive.org/details/Metallurgy_of_Steel_for_Bladesmiths_Others_who_Heat_Treat_and_Forge_Steel_By_Joh/mode/2up
Mirror: https://t.me/carbide_microstructure/236
pages 137-139
When a small particle of K1 (Cr~23~C~6~) forms in a matrix of either ferrite or austenite, the Cr atoms in the carbide are subtracted from the matrix iron immediately surrounding the particle. If this causes the %Cr to drop much below 12 %Cr in the surrounding matrix the steels become susceptible to corrosion and are said to be "sensitized". Since the carbides prefer to form along the grain boundaries of the matrix the corrosion occurs along the grain boundaries and this type of corrosion is therefore called intergranular corrosion. Hence, sensitization is said to occur when a low temperature heat treatment, such as tempering, causes K1 precipitate particle formation to the extent that intergranular corrosion may occur
He then mentions 440C to make a practical example pinpointing the fact that at 1100°C and upon quenching we will have Martensite + K2 (Cr7C3) carbides. What 440C achieves theoretically at 1100°C M390 achieves at 1200°C (15 min for a knife blade). To be picky it would be 1180°C when NOT using a vacuum furnace.
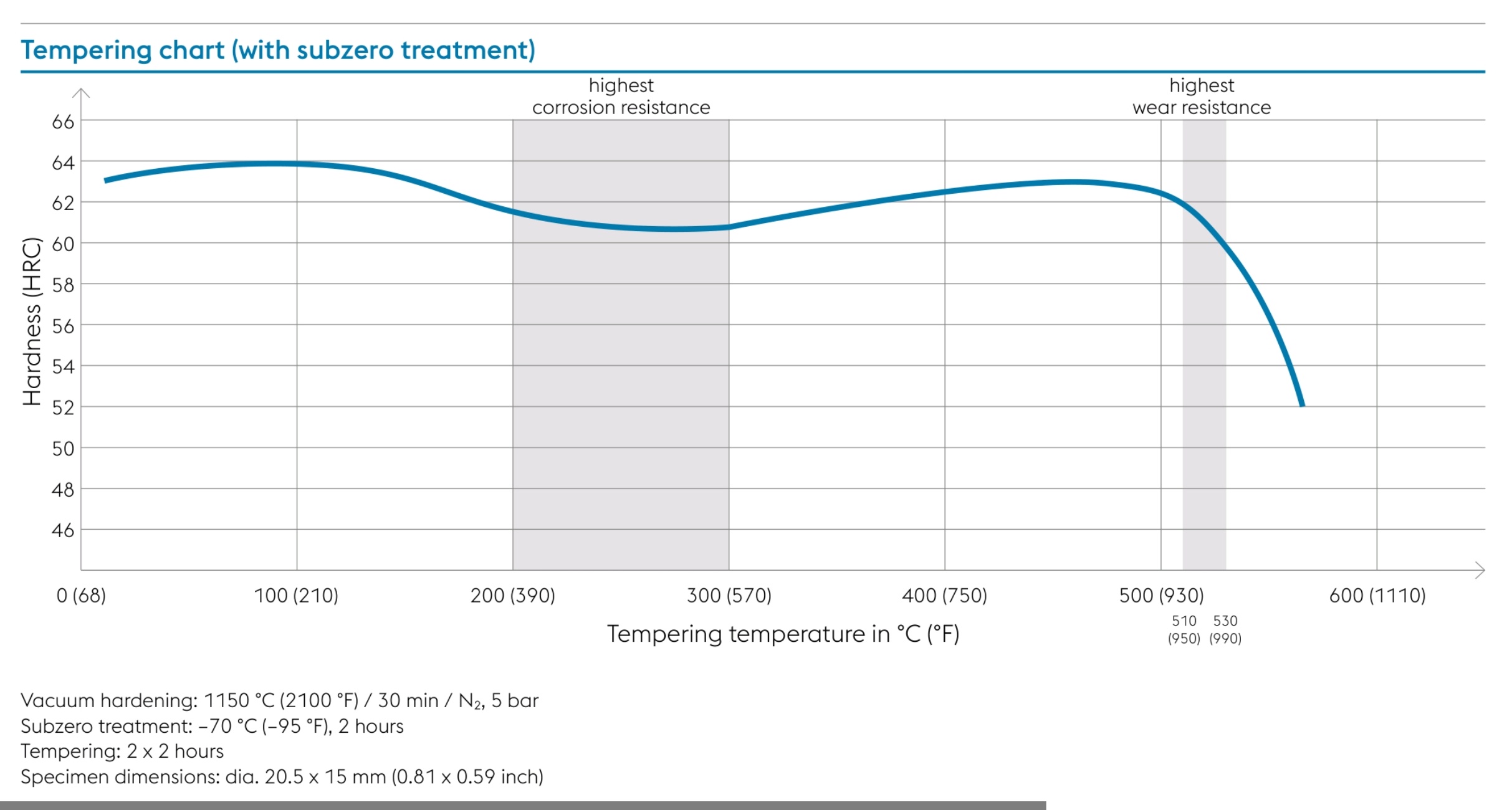
This is Bohler's tempering curve chart for m390 utilizing cryogenics to provide a more visual representation.
You can see the highest wear resistance is above the 500°c range for m390 and that the hardness does tend to drop off in that range and between 60-62 is idea (for the 1150°c austenitizing soak temperature @30 mins they have plotting the chart for, Reate's Protocol shows 1140°c and didn't specify time).
Also note that for Reate's Protocol, Reate nor Bohler didn't provide what bar pressures were used in the vacuum furnace nor the hold times. But longer and higher austenitizing soak temp hold times can create grain growth reducing performance. We can make an assumption that they are using 5bar and 30min soak times via the Bohler datasheets but thats debatable without facts not available to us.