this post was submitted on 21 Oct 2024
1278 points (98.0% liked)
Microblog Memes
5827 readers
1950 users here now
A place to share screenshots of Microblog posts, whether from Mastodon, tumblr, ~~Twitter~~ X, KBin, Threads or elsewhere.
Created as an evolution of White People Twitter and other tweet-capture subreddits.
Rules:
- Please put at least one word relevant to the post in the post title.
- Be nice.
- No advertising, brand promotion or guerilla marketing.
- Posters are encouraged to link to the toot or tweet etc in the description of posts.
Related communities:
founded 1 year ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
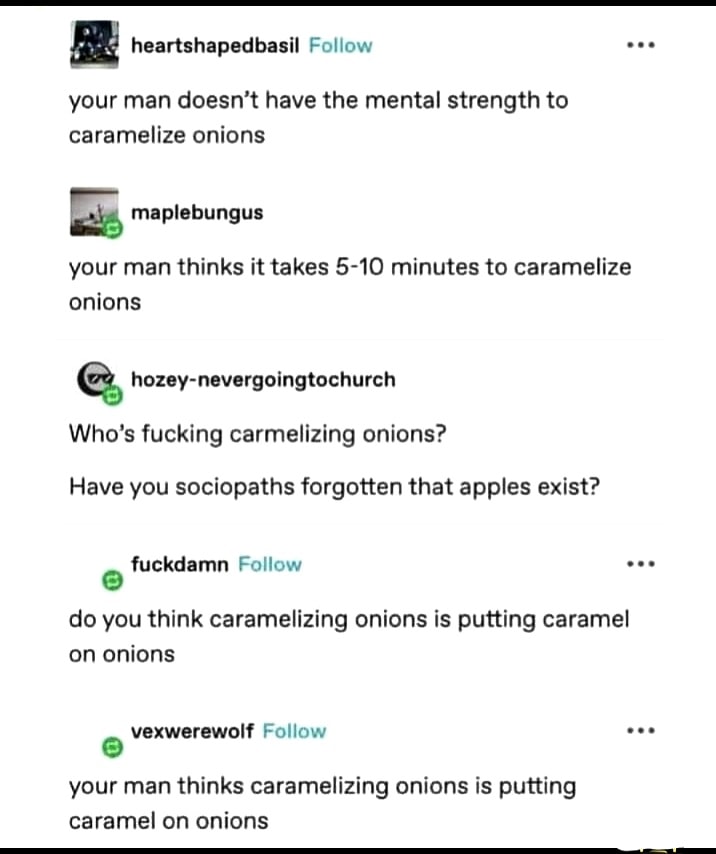
Alkalinity speeds up the Maillard reaction significantly. Baking soda. Magic.
I agree, but the comment above recommends using it to caramelize onions. Maillard reactions can happen to onions for sure but the result of that is not caramelized onions.
Not to say baking soda couldn't help, I don't know the exact chemistry behind this stuff, but I do know that onion + maillard reaction does not yield caramelized onions
Huh, I guess I'd never really looked into the chemistry behind the distinction (which is strange because i am a chemist that loves food), but Maillard reactions involve the proteins, while caramelization involves the sugars. Though both are examples of nonenzymatic browning.
The good news is that the wiki page for caramelization says that either acidic or basic conditions speed up the caramelization processes, so i think we're good to go in either front!
On that note, try adding a little splash of balsamic vinegar to caramelized onions 👌👌